- cross-posted to:
- [email protected]
- cross-posted to:
- [email protected]
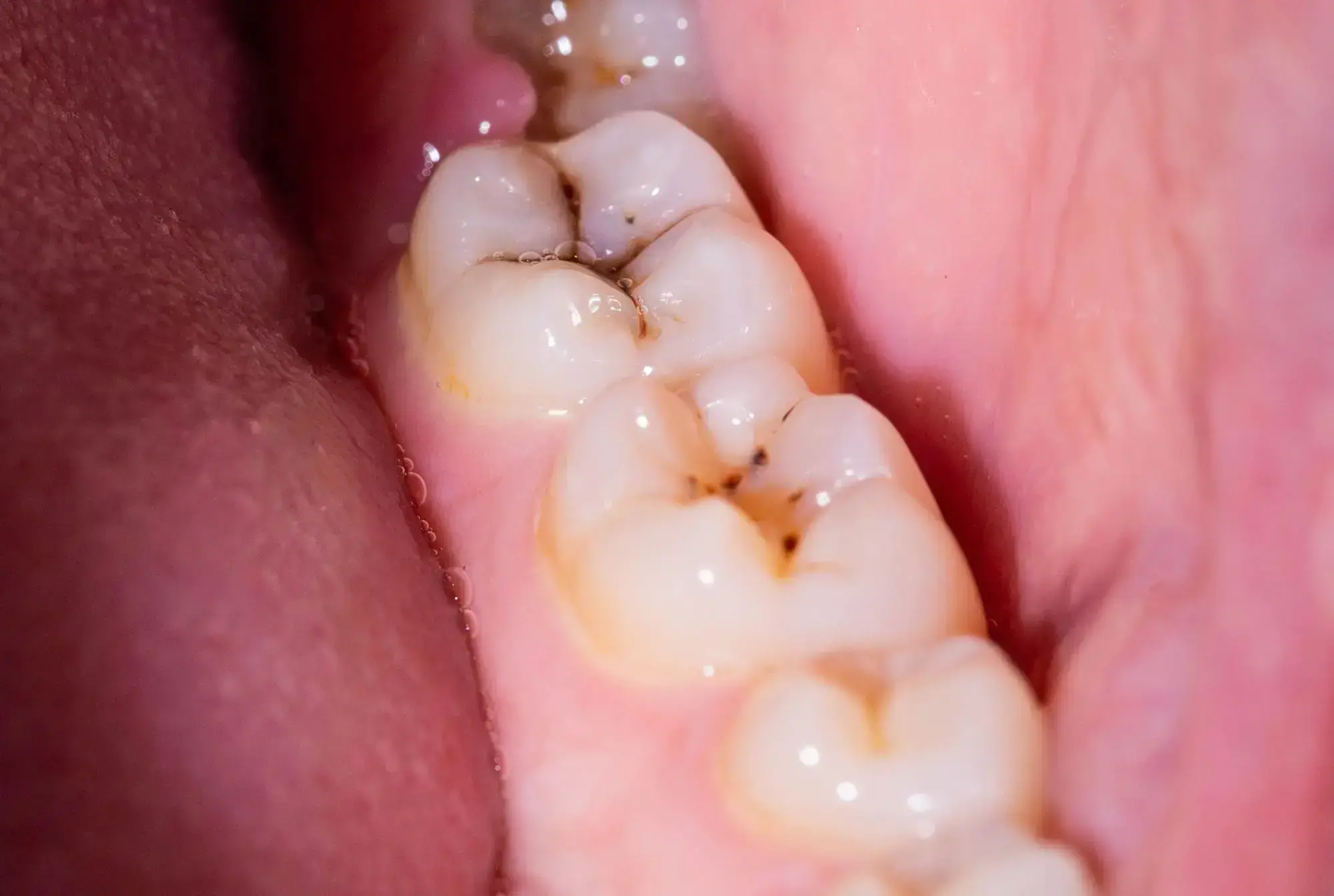
Scientists have discovered that the molecule DIM reduces biofilms causing dental plaque by 90%. Its addition to toothpaste and mouthwash could revolutionize dental hygiene. 3,3′-Diindolylmethane (DIM) decreased the Streptococcus mutans biofilm, a leading contributor to plaque and cavities, by 90%.
A significant portion of the global population experiences persistent issues with dental plaque and cavities or will face them at some time. While toothpaste, mouthwash, and routine dental visits help in prevention, there’s always room for improvement.
Researchers from Ben-Gurion University of the Negev, in collaboration with teams from Sichuan University and the National University of Singapore, have identified that 3,3′-Diindolylmethane (DIM) – a naturally occurring molecule also referred to as bisindole – can reduce biofilms responsible for plaque and cavities by a remarkable 90%.
I’ve checked the molecule in question, it’s that sort of stuff that even amateurs could make in a backyard lab, from indole and dichloromethane (use AlCl₃ as catalyst). So if the effects are real and there aren’t too big counter-effects, this will spread like wildfire.
even amateurs could make in a backyard lab, from indole and dichloromethane
I hope they don’t. Dichloromethane ain’t nothing to mess with. Wikipedia page section on its toxicity
By “even amateurs could make” I wanted to highlight how relatively simple of a molecule it is, not that they should. The impact is mostly industrial, those things that “even amateurs could make” tend to be really cheap.
With that in mind CH₂Cl₂ isn’t that big of a deal. It’s a reagent and it needs to be treated with respect and care; just like any other reagent. The worst mistakes don’t even happen with those dangerous chemicals, it’s with the ones that people think that are safe because they get sloppy with them. (Calcium carbide, glacial acetic acid, hydrogen peroxide, this sort of stuff.)
it’s with the ones that amateurs think that are safe because they get sloppy with them.
I changed and highlighted a word that I have concerns about. Keeping in mind, an amateur would have to make sure they correctly purify the end product, and make sure dichloromethane isn’t in the end product.
[DISCLAIMER: I’ll emphasise for the sake of clueless readers that Nobody should this at home. I’m talking on abstract grounds, I just wanted to highlight that DIM is easy to produce thus likely cheap. Plus another poster highlighted that the “magic” product is already industrially produced. OK? Seriously, backyard organics is not like backyard electrolysis, there are 100 ways to die out of it.]
Leftover DCM in the end product is the least concern. Purification is basically “add diluted acid”, keep the aqueous layer, discard/reuse the solvent layer. You could also boil it off to be extra sure, after crystallisation
The actual danger are the vapours, and mostly towards the amateur, not his guinea pig using this stuff. Carcinogenic, volatile, and flammable - if you try this on a Bunsen burner or a kitchen stove you’re risking some explosion, specially if you don’t ventilate this well. Amateurs have that tendency to think “this doesn’t drip on my hand, it’s invisible, so might as well not care that much about it”, and then they get wrecked.
It’s also the main component of some plastic model glues.